Smart Procurement: How to Evaluate IV Cannula Quality Beyond Just Price?
Because the Lowest Cost Can Sometimes Be the Highest Risk
For hospitals, distributors, and tenders alike, procurement of IV cannulas is one of the most recurringand seemingly straightforward tasks. You look at price quotes, check if the product is CE-marked, and pick the lowest bidder, right?
But here’s the catch: “An IV cannula isn’t just a needle and a tube. It’s a lifeline.”
And poor-quality IV cannulas don’t just lead to patient discomfort; they can cause infections, blockages, repeat insertions, and costly complications. That’s why smart procurement goes beyond price tags and into performance, safety, and consistency.
Here’s a practical guide for purchase managers, biomedical engineers, and medical distributors on how to evaluate IV cannula quality more holistically.
First: Understand the True Cost
The base price per unit (₹ or $) doesn’t tell the full story. Consider:
- Extra staff time required when cannulas block or dislodge
- Replacement cost for failed insertions
- Patient dissatisfaction and legal liability for phlebitis or infection
- Delayed therapy in critical cases
- Wasted consumables (tape, gloves, saline) per re-insertion
A low-quality cannula may cost $ less but $$$ more in clinical time.
Key Factors to Evaluate Quality
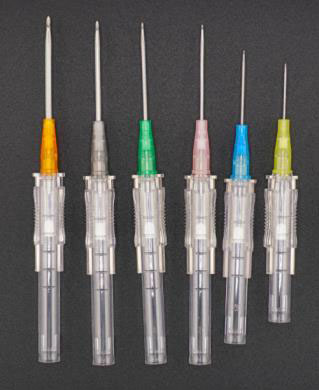
1. Needle Sharpness & Bevel Precision
A sharp, triple-faceted needle ensures:
- Less pain during insertion
- Fewer failed attempts
- Less tissue trauma and bleeding
Ask for insertion force test results or clinical feedback.
2. Flashback Chamber Visibility
A transparent flashback chamber allows the nurse to see when the vein has been accessed.
Red flags:
- Murky or opaque plastic
- Flashback not visible in under 2 seconds
Check real samples under clinical lighting.
3. Catheter Material & Glide
- High-quality FEP or PU catheters glide smoothly into the vein
- Cheap PVC or thick-walled catheters tend to kink or resist insertion
Ask for material data sheets, tensile strength tests, and clinical feedback.
4. Injection Port Quality
If your cannula includes a port, check:
- Is the valve leak-proof?
- Can it handle multiple flushes?
- Does it offer closed system access?
Do saline push tests with a syringe. Look for leaks, backflow, or resistance.
5. Sterility Assurance
Even with EO sterilization, quality can vary based on:
- Pre-sterile bioburden (cleanroom standards)
- EO exposure validation (ISO 11135)
- Packaging integrity (peel tests, visual defects)
Ask for EO residue reports and sterility validation protocols.
6. Packaging Strength & Tamper Proofing
Good packaging ensures:
- Cannulas don’t tear or puncture the blister
- Sterility isn’t compromised during transport
- Expiry and batch traceability is visible
Perform a peel test and inspect outer cartons for real-world durability.
7. Regulatory Compliance
Don’t just ask: “Is it CE-marked?”
Instead, verify:
- ISO 13485 certification
- Batch-wise CE declaration of conformity
- UDI/barcoding systems for hospital stock-keeping
Cross-verify certification numbers online or request audit reports.
Smart Procurement Checklist
Here’s a quick table to compare multiple brands beyond cost:
Feature | Brand A | Brand B | Brand C |
Needle sharpness test passed? | ✅ | ❌ | ✅ |
Flashback within 2 seconds? | ✅ | ✅ | ❌ |
EO residue below limit? | ✅ | ❌ | ✅ |
Cleanroom class certified? | ✅ | ❌ | ✅ |
Packaging peel-tested? | ✅ | ✅ | ✅ |
Certified under ISO 13485? | ✅ | ✅ | ✅ |
Price per unit | ₹7.50 | ₹6.00 | ₹8.00 |
Now ask yourself — which one truly costs more?
What Smart Buyers Do Differently
Procurement officers at top hospitals now:
- Ask for third-party test reports from NABL or equivalent labs
- Visit the factory or cleanroom virtually before large orders
- Test 5-10 cannulas from each lot before approval
- Track product complaints with structured feedback forms
- Include “functional quality” scores in tenders and not just price and CE marking
What Cubit MediSurge Offers
At Cubit, we’ve designed our IV cannulas with quality-first thinking:
- Smooth FEP catheter with excellent kink resistance
- Razor-sharp needles tested for consistent insertion force
- Crystal-clear flashback chambers
- ISO 10,000 cleanroom assembly
- EO sterilization validated to ISO 11135
- Leak-free injection ports
- Tamper-proof peel packaging with barcoded traceability
And yes, we’re competitively priced too. But we compete on reliability, not shortcuts.
In healthcare, the real cost of a product isn’t what you pay, it’s what you compromise.
So the next time a vendor gives you a quote, ask:“Can you prove your cannula is safe, consistent, and patient-friendly?”Not just “Is it the cheapest?”Because when it comes to IV access, precision matters more than pennies.
